
A medical device startup company, developing a breakthrough solution for transcatheter mitral valve replacement.
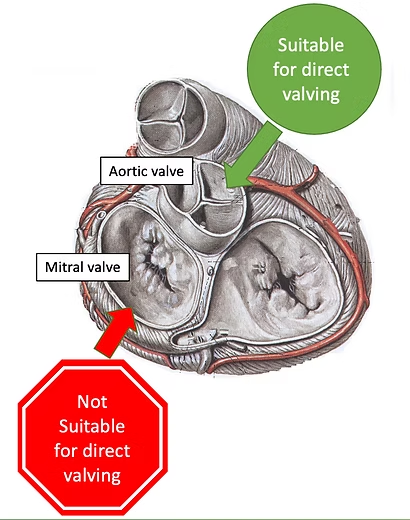
TMVR poses new challenges in comparison with TAVI:
Robust anchoring of the valve to the native annulus
Implantation in a minimally invasive approach – transcatheter
Creation of a simple and safe procedure
Elimination of paravalvular leaks (PVL)
Elimination of Left Ventricular Outflow Tract (LVOT) obstruction
Minimization of disturbance to the cardiac structure
Large valve area and high work blood pressures
Valve prosthesis durability
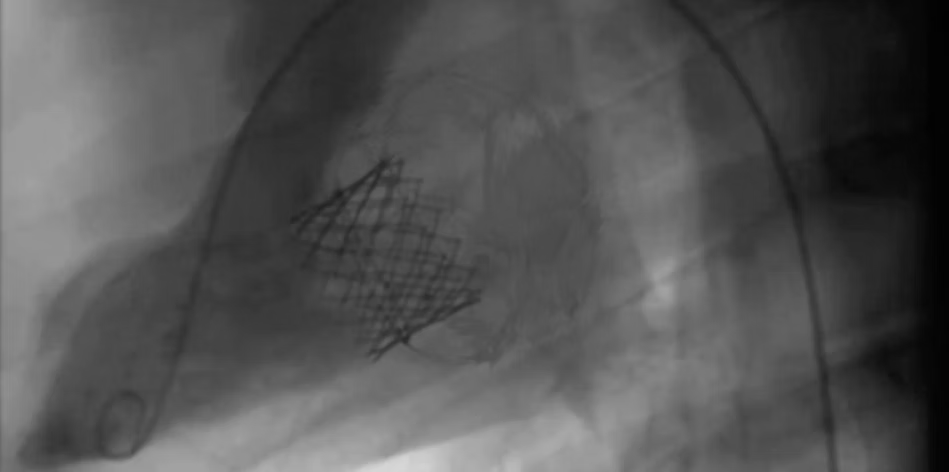
TruLeaf Medical has recently completed a successful animal trial in porcine model.
As part of the development process, the study was performed in order to demonstrate the safety and performance of the system, for an extensive time period.
See on the left: one of many successful implantations.
August 14, 2022
May 13, 2020
Allium Medical (TASE: ALMD), an Israeli medical device company, specializes in minimally invasive technologies subsidiary, TruLeaf Medical, has successfully tested a novel approach for transcatheter mitral valve replacement in patients suffering from severe mitral valve regurgitation during a long-term trial on large animals.
April 5, 2020
TruLeaf Medical has received a 5M NIS grant from the Israeli Innovation Authority. This grant follows an additional 2.6M NIS grant received during the month of March 2020.
The grant was approved for the continuation of development of a minimally invasive trans catheter mitral valve replacement. TruLeaf, a daughter company of Allium Medical, is developing a system for the replacement of the mitral valve in patients suffering from severe mitral regurgitation, inserted using catheterization techniques.
The grant will be used for furthering the R&D activities, TruLeaf’s IP and design freeze towards a first clinical study in human patients.